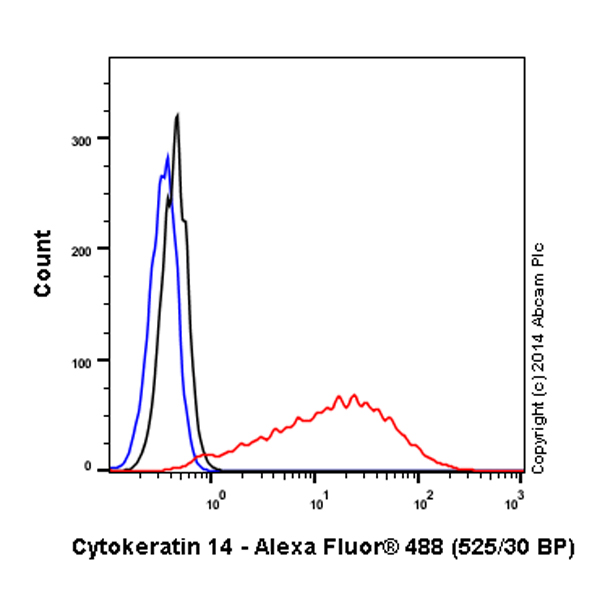
Overlay histogram showing A431 cells stained with ab192055 (red line). The cells were fixed with 80% methanol (5 min) and then permeabilized with 0.1% PBS-Triton X-100 for 20 min. The cells were then incubated in 1x PBS / 10% normal goat serum / 0.3M glycine to block non-specific protein-protein interactions followed by the antibody (ab192055, 1/500 dilution) for 30 min at 22°C. Isotype control antibody (black line) was rabbit IgG (monoclonal) Alexa Fluor® 488 used at the same concentration and conditions as the primary antibody. Unlabelled sample (blue line) was also used as a control.Acquisition of >5,000 events were collected using a 20mW Argon ion laser (488nm) and 525/30 bandpass filter.This antibody gave a positive signal in A431 fixed with 4% formaldehyde (10 min)/permeabilized with 0.1% PBS-Triton X-100 for 20 min used under the same conditions.
![ab192055 staining Cytokeratin 14 in A431 cells. The cells were fixed with 100% methanol (5min), permeabilized in 0.1% Triton X-100 for 5 minutes and then blocked in 1% BSA/10% normal goat serum/0.3M glycine in 0.1% PBS-Tween for 1h. The cells were then incubated with ab192055 at a working dilution of 1 in 100 (shown in green) and ab7291 (Mouse monoclonal [DM1A] to alpha Tubulin) at 1µg/ml overnight at +4°C, followed by a further incubation at room temperature for 1h with an Alexa Fluor® 594 Goat anti-Mouse secondary (ab150120) at 2 μg/ml (shown in red). Nuclear DNA was labelled in blue with DAPI.This product also gave a positive signal in 4% formaldehyde (10 min) fixed A431 cells under the same testing conditions.Image was taken with a Confocal microscope (Leica-microsystems, TCS SP8).](http://www.bioprodhub.com/system/product_images/ab_products/2/sub_2/5179_ab192055-233544-ab192055-ap2065433-5ug-ab7291-1ug-ab150120-2ug-A431m.jpg)
ab192055 staining Cytokeratin 14 in A431 cells. The cells were fixed with 100% methanol (5min), permeabilized in 0.1% Triton X-100 for 5 minutes and then blocked in 1% BSA/10% normal goat serum/0.3M glycine in 0.1% PBS-Tween for 1h. The cells were then incubated with ab192055 at a working dilution of 1 in 100 (shown in green) and ab7291 (Mouse monoclonal [DM1A] to alpha Tubulin) at 1µg/ml overnight at +4°C, followed by a further incubation at room temperature for 1h with an Alexa Fluor® 594 Goat anti-Mouse secondary (ab150120) at 2 μg/ml (shown in red). Nuclear DNA was labelled in blue with DAPI.This product also gave a positive signal in 4% formaldehyde (10 min) fixed A431 cells under the same testing conditions.Image was taken with a Confocal microscope (Leica-microsystems, TCS SP8).
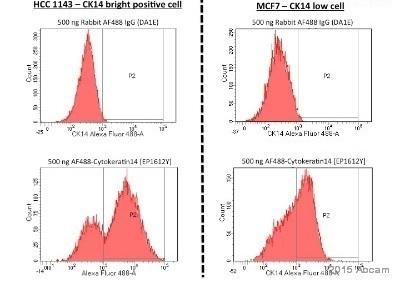
ab192055 staining Cytokeratin 14 in CK14 bright positive and low cell lines by Flow Cytometry. Cells were fixed with paraformaldehyde and permeabilized with PBS + 5% FBS + 2% Triton X-100. The sample was incubated with the primary antibody (1/50 in PBS + 5% FBS + 0.2% Triton X-100) for 45 minutes at 4°C.Gating Strategy: Doublet were excluded based on SSC - FSC profile.See Abreview

![ab192055 staining Cytokeratin 14 in A431 cells. The cells were fixed with 100% methanol (5min), permeabilized in 0.1% Triton X-100 for 5 minutes and then blocked in 1% BSA/10% normal goat serum/0.3M glycine in 0.1% PBS-Tween for 1h. The cells were then incubated with ab192055 at a working dilution of 1 in 100 (shown in green) and ab7291 (Mouse monoclonal [DM1A] to alpha Tubulin) at 1µg/ml overnight at +4°C, followed by a further incubation at room temperature for 1h with an Alexa Fluor® 594 Goat anti-Mouse secondary (ab150120) at 2 μg/ml (shown in red). Nuclear DNA was labelled in blue with DAPI.This product also gave a positive signal in 4% formaldehyde (10 min) fixed A431 cells under the same testing conditions.Image was taken with a Confocal microscope (Leica-microsystems, TCS SP8).](http://www.bioprodhub.com/system/product_images/ab_products/2/sub_2/5179_ab192055-233544-ab192055-ap2065433-5ug-ab7291-1ug-ab150120-2ug-A431m.jpg)
