![Overlay histogram showing HepG2 cells stained with ab66340 (red line). The cells were fixed with 4% paraformaldehyde (10 min) and then permeabilized with 0.1% PBS-Tween for 20 min. The cells were then incubated in 1x PBS / 10% normal goat serum / 0.3M glycine to block non-specific protein-protein interactions followed by the antibody (ab66340, 1/100 dilution) for 30 min at 22°C. The secondary antibody used was Alexa Fluor® 488 goat anti-mouse IgG (H+L) (ab150113) at 1/2000 dilution for 30 min at 22°C. Isotype control antibody (black line) was mouse IgG1 [ICIGG1] (ab91353, 1μg/1x106 cells) used under the same conditions. Unlabelled sample (blue line) was also used as a control. Acquisition of >5,000 events were collected using a 20mW Argon ion laser (488nm) and 525/30 bandpass filter. This antibody gave a positive signal in HepG2 cells fixed with 80% methanol (5 min)/permeabilized with 0.1% PBS-Tween for 20 min used under the same conditions.](http://www.bioprodhub.com/system/product_images/ab_products/2/sub_2/25101_ab66340-5-ab66340FC.jpg)
Overlay histogram showing HepG2 cells stained with ab66340 (red line). The cells were fixed with 4% paraformaldehyde (10 min) and then permeabilized with 0.1% PBS-Tween for 20 min. The cells were then incubated in 1x PBS / 10% normal goat serum / 0.3M glycine to block non-specific protein-protein interactions followed by the antibody (ab66340, 1/100 dilution) for 30 min at 22°C. The secondary antibody used was Alexa Fluor® 488 goat anti-mouse IgG (H+L) (ab150113) at 1/2000 dilution for 30 min at 22°C. Isotype control antibody (black line) was mouse IgG1 [ICIGG1] (ab91353, 1μg/1x106 cells) used under the same conditions. Unlabelled sample (blue line) was also used as a control. Acquisition of >5,000 events were collected using a 20mW Argon ion laser (488nm) and 525/30 bandpass filter. This antibody gave a positive signal in HepG2 cells fixed with 80% methanol (5 min)/permeabilized with 0.1% PBS-Tween for 20 min used under the same conditions.
![All lanes : Anti-Glucose 6 phosphate isomerase antibody [1B7D7] (ab66340) at 1/2000 dilutionLane 1 : Cell lysates prepared from HepG2 cells.Lane 2 : Cell lysates prepared from SMMC-7721 cells.Lysates/proteins at 100 µg per lane.SecondaryHRP-conjugated Goat polyclonal to mouse IgG1](http://www.bioprodhub.com/system/product_images/ab_products/2/sub_2/25102_Glucose-6-phosphate-isomerase-Primary-antibodies-ab66340-2.jpg)
All lanes : Anti-Glucose 6 phosphate isomerase antibody [1B7D7] (ab66340) at 1/2000 dilutionLane 1 : Cell lysates prepared from HepG2 cells.Lane 2 : Cell lysates prepared from SMMC-7721 cells.Lysates/proteins at 100 µg per lane.SecondaryHRP-conjugated Goat polyclonal to mouse IgG1
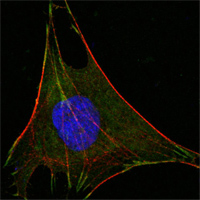
ab66340 at 1000 dilution staining Glucose 6 phosphate isomerase in L-02 cells by Immunocytochemistry/ Immunofluorescence. An Alexa Fluor® 488 conjugated Goat polyclonal to mouse IgG1 was used as secondary antibody. Green staining in image show positive staining with ab66340, actin filaments were stained red with DY-554 phalloidin and nuclei stained blue with DRAQ5 flourescent DNA dye.
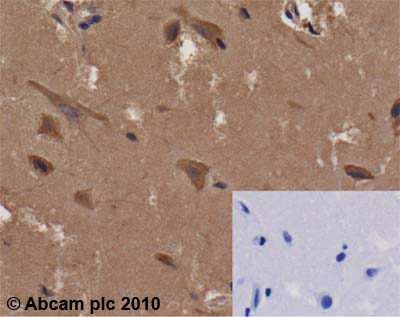
ab66340 (1µg/ml) staining Glucose 6 phosphate isomerase in human cerebral cortex using an automated system (DAKO Autostainer Plus). Using this protocol there is strong cytoplasmic staining of neurons and of the neuropil.Inset panel depicts negative control (no primary antibody).Sections were rehydrated and antigen retrieved with the Dako 3 in 1 AR buffer EDTA pH 9.0 in a DAKO PT link. Slides were peroxidase blocked in 3% H2O2 in methanol for 10 mins. They were then blocked with Dako Protein block for 10 minutes (containing casein 0.25% in PBS) then incubated with primary antibody for 20 min and detected with Dako envision flex amplification kit for 30 minutes. Colorimetric detection was completed with Diaminobenzidine for 5 minutes. Slides were counterstained with Haematoxylin and coverslipped under DePeX. Please note that, for manual staining, optimization of primary antibody concentration and incubation time is recommended. Signal amplification may be required.
![Overlay histogram showing HepG2 cells stained with ab66340 (red line). The cells were fixed with 4% paraformaldehyde (10 min) and then permeabilized with 0.1% PBS-Tween for 20 min. The cells were then incubated in 1x PBS / 10% normal goat serum / 0.3M glycine to block non-specific protein-protein interactions followed by the antibody (ab66340, 1/100 dilution) for 30 min at 22°C. The secondary antibody used was Alexa Fluor® 488 goat anti-mouse IgG (H+L) (ab150113) at 1/2000 dilution for 30 min at 22°C. Isotype control antibody (black line) was mouse IgG1 [ICIGG1] (ab91353, 1μg/1x106 cells) used under the same conditions. Unlabelled sample (blue line) was also used as a control. Acquisition of >5,000 events were collected using a 20mW Argon ion laser (488nm) and 525/30 bandpass filter. This antibody gave a positive signal in HepG2 cells fixed with 80% methanol (5 min)/permeabilized with 0.1% PBS-Tween for 20 min used under the same conditions.](http://www.bioprodhub.com/system/product_images/ab_products/2/sub_2/25101_ab66340-5-ab66340FC.jpg)
![All lanes : Anti-Glucose 6 phosphate isomerase antibody [1B7D7] (ab66340) at 1/2000 dilutionLane 1 : Cell lysates prepared from HepG2 cells.Lane 2 : Cell lysates prepared from SMMC-7721 cells.Lysates/proteins at 100 µg per lane.SecondaryHRP-conjugated Goat polyclonal to mouse IgG1](http://www.bioprodhub.com/system/product_images/ab_products/2/sub_2/25102_Glucose-6-phosphate-isomerase-Primary-antibodies-ab66340-2.jpg)

