ab2893 staining γH2A.X in MALME-3M cells treated with terfenadine (ab120270), by ICC/IF. Increase of γH2A.X nuclear expression correlates with increased concentration of terfenadine, as described in literature.The cells were incubated at 37°C for 6 hours in media containing different concentrations of ab120270 (terfenadine) in DMSO, fixed with 4% formaldehyde for 10 minutes at room temperature and blocked with PBS containing 10% goat serum, 0.3 M glycine, 1% BSA and 0.1% tween for 2h at room temperature. Staining of the treated cells with ab2893 (10 μg/ml) was performed overnight at 4°C in PBS containing 1% BSA and 0.1% tween. A DyLight 488 anti-rabbit polyclonal antibody (ab96899) at 1/250 dilution was used as the secondary antibody. Nuclei were counterstained with DAPI and are shown in blue.
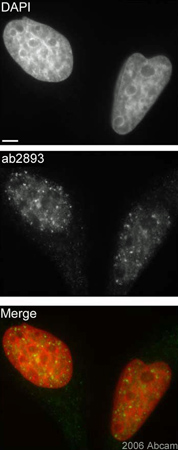
Asynchronous HeLa cells were exposed to 2Gy and permitted to recover for 30min. Cells were paraformaldehyde fixed (4%), immunofluorescently labeled with ab2893 and counterstained with DAPI. The merge image presents the DAPI and ab2893 channels as red and green, respectively. The scale bar represents 5µm.
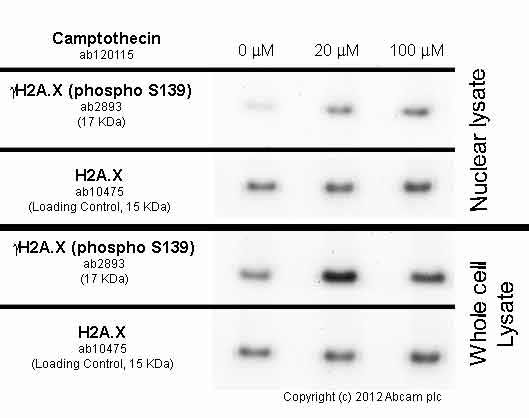
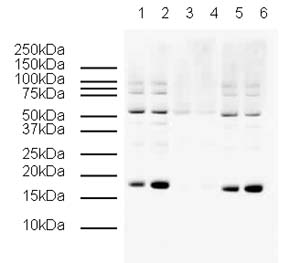
developed using the ECL techniquePerformed under reducing conditions.

Asynchronous HeLa cells were paraformaldehyde fixed and immunofluorescently labeled with ab2893 that had been preincubated with either 1) non-phosphorylated or 2) phosphorylated H2AX peptide. Identical exposure times were employed. The Merge images present the DAPI and ab2893 channels as red and green, respectively. Scale bars represent 5µm.1) Non-phosphorylated peptides2) Phosphorylated peptides
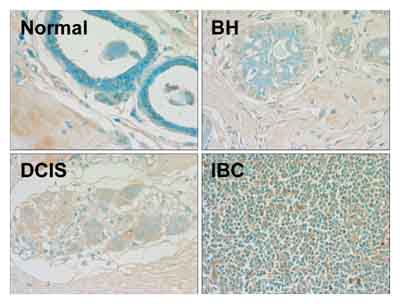
ab2893 staining gamma H2A.X in human mammary tissue by Immunohistochemistry (paraffin embedded sections).Paraffin-embedded blocks were sectioned and mounted on frost-free slides. The 3-10 µm sections were deparaffinized in xylene and rehydrated through a series of graded alcohols. Slides were washed with 1× PBS and endogenous peroxidases were blocked with 1.5% hydrogen peroxide in 1× PBS for 20 minutes at 25°C. After three 5 minutes washes in 1× PBS, slides were incubated in blocking solution (1× PBS with 0.1% Triton X-100, 3% bovine serum albumin) with 5% normal donkey serum for 10 minutes at 25°C. Control (no primary antibody) and experimental slides were incubated overnight at 4°C, respectively, in blocking solution alone or blocking solution with ab2893 at 1/1000 dilution. Biotin-conjugated secondary antibody 1/200 was added and slides were incubated at 25°C for 30 minutes and then washed three times with 1× PBS. The ABC Peroxidase Staining kit (1/100 dilution of each Rea
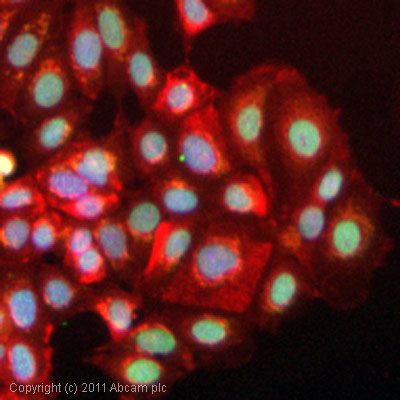
ICC/IF image of ab2893 stained MCF7 cells. The cells were 100% methanol fixed (5 min) and then incubated in 1%BSA / 10% normal goat serum / 0.3M glycine in 0.1% PBS-Tween for 1h to permeabilise the cells and block non-specific protein-protein interactions. The cells were then incubated with the antibody (ab2893, 5µg/ml) overnight at +4°C. The secondary antibody (green) was DyLight® 488 goat anti-rabbit IgG - H&L, pre-adsorbed (ab96899) used at a 1/250 dilution for 1h. Alexa Fluor® 594 WGA was used to label plasma membranes (red) at a 1/200 dilution for 1h. DAPI was used to stain the cell nuclei (blue) at a concentration of 1.43µM.
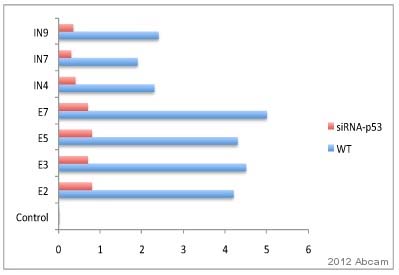
ab2893 used in ChIP.Nuclear lysate prepared from murine testis cells (irradiated with 20 Gy).Cross-linking (X-ChIP) 15 minutes.Detection step: Real-time PCR.ab2893 used at a 1/50 dilution.See Abreview
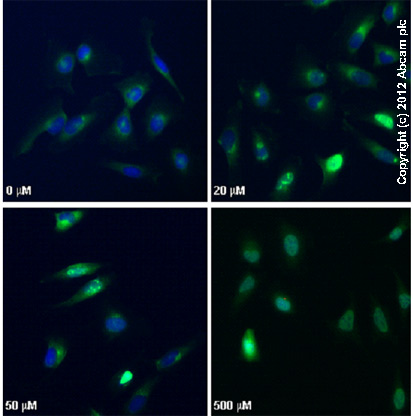
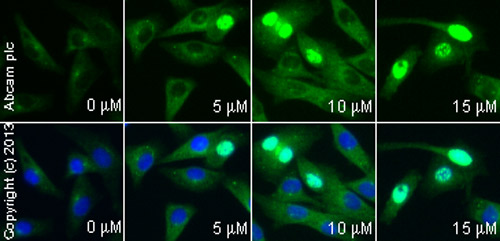
ab2893 staining γH2AX (phospho S139) in HeLa cells treated with camptothecin (ab120115), by ICC/IF. Increased nuclear expression of γH2AX (phospho S139) correlates with increased concentration of camptothecin, as described in literature.The cells were incubated at 37°C for 3h in media containing different concentrations of ab120115 (camptothecin) in DMSO, fixed with 4% formaldehyde for 10 minutes at room temperature and blocked with PBS containing 10% goat serum, 0.3 M glycine, 1% BSA and 0.1% tween for 2h at room temperature. Staining of the treated cells with ab2893 (10 µg/ml) was performed overnight at 4°C in PBS containing 1% BSA and 0.1% tween. A DyLight 488 goat anti-rabbit polyclonal antibody (ab96899) at 1/250 dilution was used as the secondary antibody. Nuclei were counterstained with DAPI and are shown in blue.
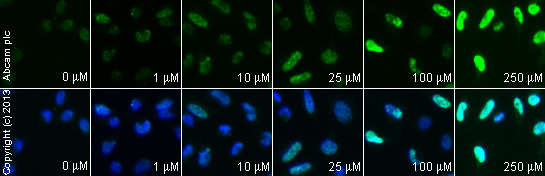
ab2893 staining γH2A.X in HeLa cells treated with SN 38 (ab141108), by ICC/IF. Increase of γH2A.X nuclear expression correlates with increased concentration of SN 38, as described in literature.The cells were incubated at 37°C for 6 hours in media containing different concentrations of ab141108 (SN 38) in DMSO, fixed with 100% methanol for 5 minutes at -20°C and blocked with PBS containing 10% goat serum, 0.3 M glycine, 1% BSA and 0.1% tween for 2h at room temperature. Staining of the treated cells with ab2893 (5 µg/ml) was performed overnight at 4°C in PBS containing 1% BSA and 0.1% tween. A DyLight 488 anti-rabbit polyclonal antibody (ab96899) at 1/250 dilution was used as the secondary antibody. Nuclei were counterstained with DAPI and are shown in blue.
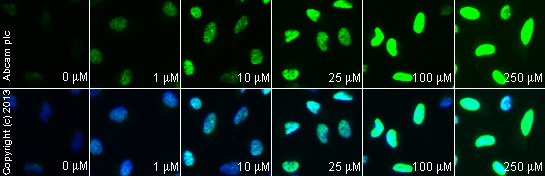
ab2893 staining γH2A.X in HeLa cells treated with CPT 11 (Irinotecan) (ab141107), by ICC/IF. Increase of γH2A.X nuclear expression correlates with increased concentration of CPT 11 (Irinotecan), as described in literature.The cells were incubated at 37°C for 6 hours in media containing different concentrations of ab141107 (CPT 11 (Irinotecan)) in DMSO, fixed with 100% methanol for 5 minutes at -20°C and blocked with PBS containing 10% goat serum, 0.3 M glycine, 1% BSA and 0.1% tween for 2h at room temperature. Staining of the treated cells with ab2893 (5 µg/ml) was performed overnight at 4°C in PBS containing 1% BSA and 0.1% tween. A DyLight 488 anti-rabbit polyclonal antibody (ab96899) at 1/250 dilution was used as the secondary antibody. Nuclei were counterstained with DAPI and are shown in blue.
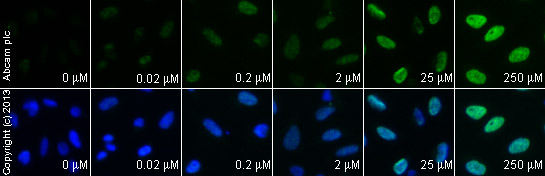
ab2893 staining γH2A.X in HeLa cells treated with 10-Hydroxycamptothecin (ab141071), by ICC/IF. Increase of γH2A.X nuclear expression correlates with increased concentration of 10-Hydroxycamptothecin, as described in literature.The cells were incubated at 37°C for 6 hours in media containing different concentrations of ab141071 (10-Hydroxycamptothecin) in DMSO, fixed with 100% methanol for 5 minutes at -20°C and blocked with PBS containing 10% goat serum, 0.3 M glycine, 1% BSA and 0.1% tween for 2h at room temperature. Staining of the treated cells with ab2893 (5 µg/ml) was performed overnight at 4°C in PBS containing 1% BSA and 0.1% tween. A DyLight 488 anti-rabbit polyclonal antibody (ab96899) at 1/250 dilution was used as the secondary antibody. Nuclei were counterstained with DAPI and are shown in blue.
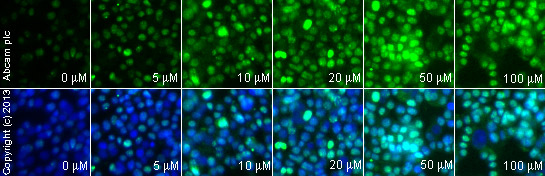
ab2893 staining γH2A.X in weri cells treated with TMPyP4 tosylate (ab120793), by ICC/IF. Increase of γH2A.X nuclear expression correlates with increased concentration of TMPyP4 tosylate, as described in literature.The cells were incubated at 37°C for 24 hours in media containing different concentrations of ab120793 (TMPyP4 tosylate ) in DMSO, fixed with 4% formaldehyde for 10 minutes at room temperature and blocked with PBS containing 10% goat serum, 0.3 M glycine, 1% BSA and 0.1% tween for 2h at room temperature. Staining of the treated cells with ab2893 (1 μg/ml) was performed overnight at 4°C in PBS containing 1% BSA and 0.1% tween. A DyLight 488 anti-rabbit polyclonal antibody (ab96899) at 1/250 dilution was used as the secondary antibody. Nuclei were counterstained with DAPI and are shown in blue.