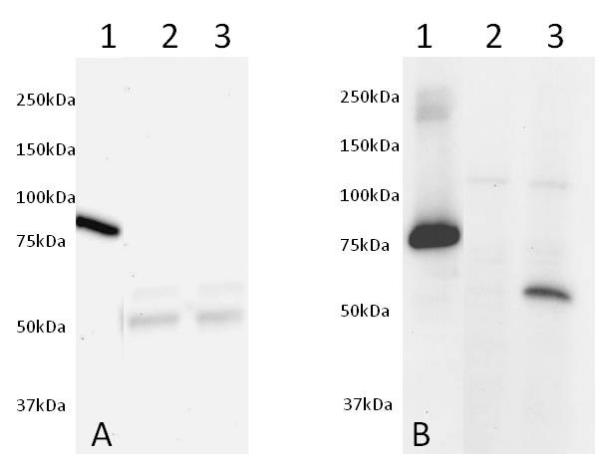
Western blot was run on a 10-20% gradient acrylamide gel. Samples were loaded as follows from left to right: (1) 50ng of Human recombinant AKT1 protein (tagged) Cat# ab62279, (2) 25µg of non-induced NIH3T3 cell extract and (3) 25µg of PDGF induced NIH3T3 cell extract. Membrane Blocking was carried out with 5% Milk+50mM Tris+0.05% Tween-20 pH 7.4, primary antibodies (A) Total AKT1 and (B) pSer473, were incubated overnight in 5% BSA+50mM+0.05% Tween-20 pH 7.4 and secondary antibodies were incubated for 2 hours in 5% Milk+50mM Tris+0.05% Tween-20 pH 7.4.
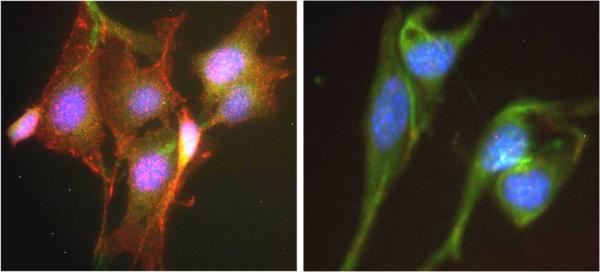
ICC was carried out on NIH3T3 cells treated with PDGF (Left) or vehicle (right) using this kit. Labeling was carried out with a polyclonal antibody GAR-594 (Akt-1 pSer473) and GAM-488 (Akt-1 total). The PDGF induced cells (left) show a significant induction of Akt phosphorylation at residue S473 in comparison to the non-induced control (right).
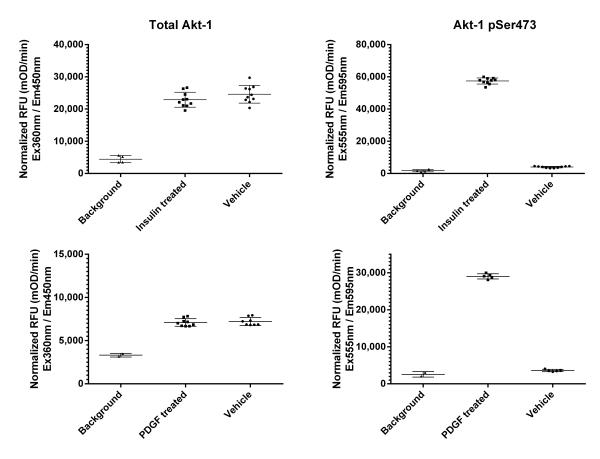
ICE was carried out on MCF7 cells seeded at 25,000 cells per well (top) and NIH3T3 cells seeded at 150,000 cells per well (bottom) treated for 30 min with appropriate compounds after overnight serum starvation. Signal is shown as a 60 minutes kinetic readout after normalization with janus green. Insulin treatment increases specific phosphorylation by 25 fold in MCF7 cells, whereas PDGF treatment increases specific phosphorylation of AKT by 50 fold in NIH3T3 cells.
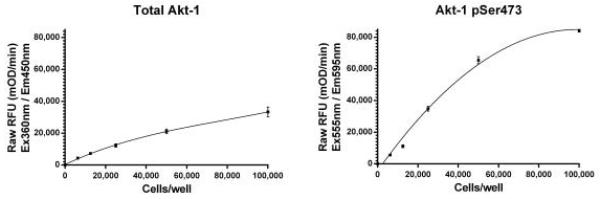
Cells were seeded the day before at the specified cell densities, allowed to adhere and exposed to serum starvation overnight. The next day, cells were treated with 50nM of Insulin for 30 minutes at 37°C. The signal was obtained using this kit as described. Total Akt1 with Ex/Em=360/450nm (LEFT) and Akt1 pSer473 with Ex/Em=555/595nm (RIGHT) are shown as raw signal after background subtraction (no primary antibody on wells seeded at corresponding density).



