Anti-ATP5A antibody [15H4C4] - Mitochondrial Marker
| Name | Anti-ATP5A antibody [15H4C4] - Mitochondrial Marker |
|---|---|
| Supplier | Abcam |
| Catalog | ab14748 |
| Prices | $404.00 |
| Sizes | 100 µg |
| Host | Mouse |
| Clonality | Monoclonal |
| Isotype | IgG2b |
| Clone | 15H4C4 |
| Applications | WB ICC/IF IP IHC-P ICC/IF ICC/IF FC |
| Species Reactivities | Mouse, Rat, Bovine, Human, C. elegans, Drosophila, Monkey |
| Antigen | Purified mitochondrial Complex V (Cow) |
| Description | Mouse Monoclonal |
| Gene | ATP5A1 |
| Conjugate | Unconjugated |
| Supplier Page | Shop |
Product images
Product References
Green tea epigallocatechin gallate binds to and inhibits respiratory complexes in - Green tea epigallocatechin gallate binds to and inhibits respiratory complexes in
Weng Z, Zhou P, Salminen WF, Yang X, Harrill AH, Cao Z, Mattes WB, Mendrick DL, Shi Q. Biochem Biophys Res Commun. 2014 Jan 17;443(3):1097-104. doi:
GCN5-like protein 1 (GCN5L1) controls mitochondrial content through coordinated - GCN5-like protein 1 (GCN5L1) controls mitochondrial content through coordinated
Scott I, Webster BR, Chan CK, Okonkwo JU, Han K, Sack MN. J Biol Chem. 2014 Jan 31;289(5):2864-72.
Metabolic enzyme expression highlights a key role for MTHFD2 and the - Metabolic enzyme expression highlights a key role for MTHFD2 and the
Nilsson R, Jain M, Madhusudhan N, Sheppard NG, Strittmatter L, Kampf C, Huang J, Asplund A, Mootha VK. Nat Commun. 2014;5:3128.
Autophagy signaling in skeletal muscle of infarcted rats. - Autophagy signaling in skeletal muscle of infarcted rats.
Jannig PR, Moreira JB, Bechara LR, Bozi LH, Bacurau AV, Monteiro AW, Dourado PM, Wisloff U, Brum PC. PLoS One. 2014 Jan 10;9(1):e85820.
A novel fizzy/Cdc20-dependent mechanism suppresses necrosis in neural stem cells. - A novel fizzy/Cdc20-dependent mechanism suppresses necrosis in neural stem cells.
Kuang C, Golden KL, Simon CR, Damrath J, Buttitta L, Gamble CE, Lee CY. Development. 2014 Apr;141(7):1453-64.
Acidosis overrides oxygen deprivation to maintain mitochondrial function and cell - Acidosis overrides oxygen deprivation to maintain mitochondrial function and cell
Khacho M, Tarabay M, Patten D, Khacho P, MacLaurin JG, Guadagno J, Bergeron R, Cregan SP, Harper ME, Park DS, Slack RS. Nat Commun. 2014 Apr 1;5:3550.
CLYBL is a polymorphic human enzyme with malate synthase and beta-methylmalate - CLYBL is a polymorphic human enzyme with malate synthase and beta-methylmalate
Strittmatter L, Li Y, Nakatsuka NJ, Calvo SE, Grabarek Z, Mootha VK. Hum Mol Genet. 2014 May 1;23(9):2313-23.
PINK1-mediated phosphorylation of Parkin boosts Parkin activity in Drosophila. - PINK1-mediated phosphorylation of Parkin boosts Parkin activity in Drosophila.
Shiba-Fukushima K, Inoshita T, Hattori N, Imai Y. PLoS Genet. 2014 Jun 5;10(6):e1004391.
An uncoupling channel within the c-subunit ring of the F1FO ATP synthase is the - An uncoupling channel within the c-subunit ring of the F1FO ATP synthase is the
Alavian KN, Beutner G, Lazrove E, Sacchetti S, Park HA, Licznerski P, Li H, Nabili P, Hockensmith K, Graham M, Porter GA Jr, Jonas EA. Proc Natl Acad Sci U S A. 2014 Jul 22;111(29):10580-5. doi:
Drosophila Syd-1, liprin-alpha, and protein phosphatase 2A B' subunit Wrd - Drosophila Syd-1, liprin-alpha, and protein phosphatase 2A B' subunit Wrd
Li L, Tian X, Zhu M, Bulgari D, Bohme MA, Goettfert F, Wichmann C, Sigrist SJ, Levitan ES, Wu C. J Neurosci. 2014 Jun 18;34(25):8474-87.
![All lanes : Anti-ATP5A antibody [15H4C4] - Mitochondrial Marker (ab14748) at 1 µg/mlLane 1 : Isolated mitochondria from human heart at 10 µgLane 2 : Isolated mitochondria from bovine heart at 4 µgLane 3 : Isolated mitochondria from rat heart at 10 µgLane 4 : Isolated mitochondria from mouse heart at 10 µgLane 5 : HepG2 lysate at 20 µg](http://www.bioprodhub.com/system/product_images/ab_products/2/sub_1/11035_ATP5A-Primary-antibodies-ab14748-12.jpg)
![All lanes : Anti-ATP5A antibody [15H4C4] - Mitochondrial Marker (ab14748) at 1 µg/mlLane 1 : Liver (Human) Tissue Lysate - adult normal tissue (ab29889)Lane 2 : HepG2 (Human hepatocellular liver carcinoma cell line) Whole Cell Lysate Lysates/proteins at 10 µg per lane.SecondaryGoat polyclonal to Mouse IgG - H&L - Pre-Adsorbed (HRP) at 1/3000 dilutionObserved band size : 55 kDa (why is the actual band size different from the predicted?)Additional bands at : 36 kDa. We are unsure as to the identity of these extra bands.](http://www.bioprodhub.com/system/product_images/ab_products/2/sub_1/11036_ATP5A-Primary-antibodies-ab14748-2.jpg)
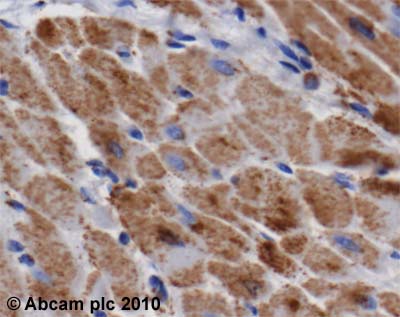
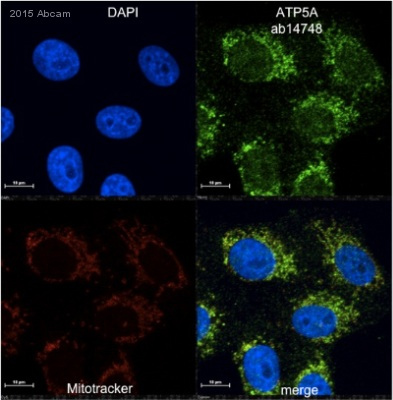
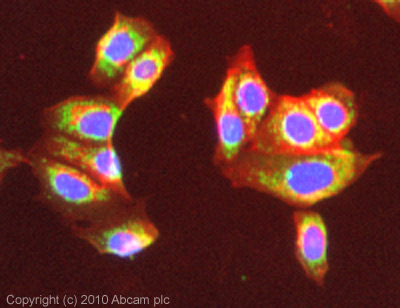
![Overlay histogram showing HepG2 cells stained with ab14748 (red line). The cells were fixed with 4% paraformaldehyde (10 min) and then permeabilized with 0.1% PBS-Tween for 20 min. The cells were then incubated in 1x PBS / 10% normal goat serum / 0.3M glycine to block non-specific protein-protein interactions followed by the antibody (ab14748, 1µg/1x106 cells) for 30 min at 22ºC. The secondary antibody used was DyLight® 488 goat anti-mouse IgG (H+L) (ab96879) at 1/500 dilution for 30 min at 22ºC. Isotype control antibody (black line) was mouse IgG2b [PLPV219] (ab91366, 2µg/1x106 cells) used under the same conditions. Acquisition of >5,000 events was performed. This antibody gave a positive signal in HepG2 cells fixed with 80% methanol (5 min)/permeabilized in 0.1% PBS-Tween used under the same conditions.](http://www.bioprodhub.com/system/product_images/ab_products/2/sub_1/11040_ATP5A-Primary-antibodies-ab14748-9.jpg)