Anti-alpha Adaptin antibody [AP6]
| Name | Anti-alpha Adaptin antibody [AP6] |
|---|---|
| Supplier | Abcam |
| Catalog | ab2730 |
| Prices | $403.00 |
| Sizes | 100 µl |
| Host | Mouse |
| Clonality | Monoclonal |
| Isotype | IgG1 |
| Clone | AP6 |
| Applications | ICC/IF ICC/IF ICC/IF B/N Electron microscopy ELISA B/N IP WB IHC-F FC |
| Species Reactivities | Mouse, Rat, Hamster, Bovine, Human, Drosophila, Primate |
| Antigen | Other Immunogen Type corresponding to alpha Adaptin |
| Description | Mouse Monoclonal |
| Gene | AP2A2 |
| Conjugate | Unconjugated |
| Supplier Page | Shop |
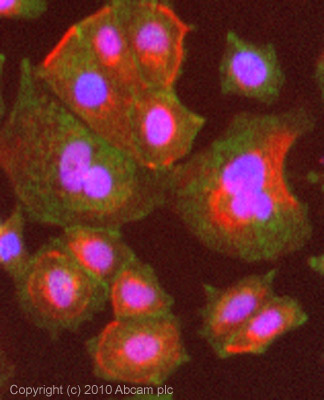
Product images
Product References
Cdc42 and the Rho GEF intersectin-1 collaborate with Nck to promote - Cdc42 and the Rho GEF intersectin-1 collaborate with Nck to promote
Humphries AC, Donnelly SK, Way M. J Cell Sci. 2014 Feb 1;127(Pt 3):673-85.
Actin and dynamin2 dynamics and interplay during clathrin-mediated endocytosis. - Actin and dynamin2 dynamics and interplay during clathrin-mediated endocytosis.
Grassart A, Cheng AT, Hong SH, Zhang F, Zenzer N, Feng Y, Briner DM, Davis GD, Malkov D, Drubin DG. J Cell Biol. 2014 Jun 9;205(5):721-35.
Cis and trans regulatory mechanisms control AP2-mediated B cell receptor - Cis and trans regulatory mechanisms control AP2-mediated B cell receptor
Busman-Sahay K, Drake L, Sitaram A, Marks M, Drake JR. PLoS One. 2013;8(1):e54938.
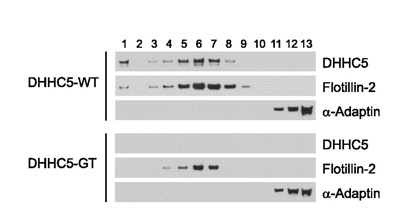
DHHC5 protein palmitoylates flotillin-2 and is rapidly degraded on induction of - DHHC5 protein palmitoylates flotillin-2 and is rapidly degraded on induction of
Li Y, Martin BR, Cravatt BF, Hofmann SL. J Biol Chem. 2012 Jan 2;287(1):523-30.
Endocytosis regulates cell soma translocation and the distribution of adhesion - Endocytosis regulates cell soma translocation and the distribution of adhesion
Shieh JC, Schaar BT, Srinivasan K, Brodsky FM, McConnell SK. PLoS One. 2011 Mar 22;6(3):e17802.
Crystal structure of nucleotide-free dynamin. - Crystal structure of nucleotide-free dynamin.
Faelber K, Posor Y, Gao S, Held M, Roske Y, Schulze D, Haucke V, Noe F, Daumke O. Nature. 2011 Sep 18;477(7366):556-60.
Clathrin mediates integrin endocytosis for focal adhesion disassembly in - Clathrin mediates integrin endocytosis for focal adhesion disassembly in
Ezratty EJ, Bertaux C, Marcantonio EE, Gundersen GG. J Cell Biol. 2009 Nov 30;187(5):733-47.
Molecular basis for the sorting of the SNARE VAMP7 into endocytic clathrin-coated - Molecular basis for the sorting of the SNARE VAMP7 into endocytic clathrin-coated
Pryor PR, Jackson L, Gray SR, Edeling MA, Thompson A, Sanderson CM, Evans PR, Owen DJ, Luzio JP. Cell. 2008 Sep 5;134(5):817-27.
Rho/ROCK and myosin II control the polarized distribution of endocytic clathrin - Rho/ROCK and myosin II control the polarized distribution of endocytic clathrin
Samaniego R, Sanchez-Martin L, Estecha A, Sanchez-Mateos P. J Cell Sci. 2007 Oct 15;120(Pt 20):3534-43. Epub 2007 Sep 25.
Transmitter release face Ca2+ channel clusters persist at isolated presynaptic - Transmitter release face Ca2+ channel clusters persist at isolated presynaptic
Sun L, Li Q, Khanna R, Chan AW, Wong F, Stanley EF. Eur J Neurosci. 2006 Mar;23(5):1391-6.
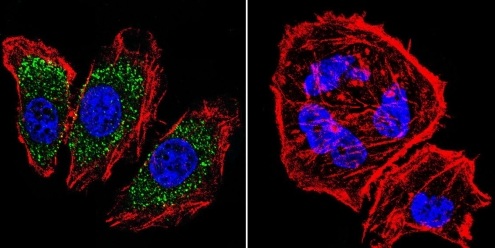
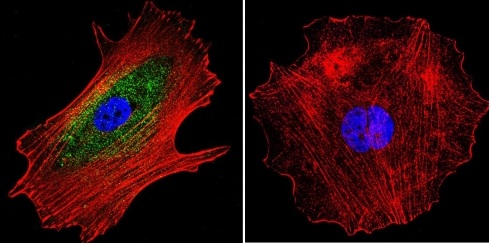
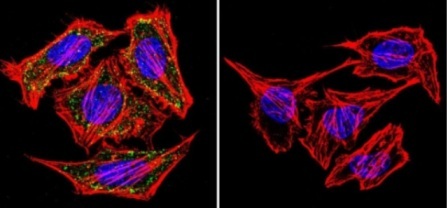
![Overlay histogram showing MCF7 cells stained with ab2730 (red line). The cells were fixed with 4% paraformaldehyde (10 min) and then permeabilized with 0.1% PBS-Tween for 20 min. The cells were then incubated in 1x PBS / 10% normal goat serum / 0.3M glycine to block non-specific protein-protein interactions followed by the antibody (ab2730, 1µg/1x106 cells) for 30 min at 22ºC. The secondary antibody used was a goat anti-mouse DyLight® 488 (IgG; H+L) (ab96879) at 1/500 dilution for 30 min at 22ºC. Isotype control antibody (black line) was mouse IgG1 [ICIGG1] (ab91353, 2µg/1x106 cells) used under the same conditions. Acquisition of >5,000 events was performed.](http://www.bioprodhub.com/system/product_images/ab_products/2/sub_1/5626_alpha-Adaptin-Primary-antibodies-ab2730-3.jpg)