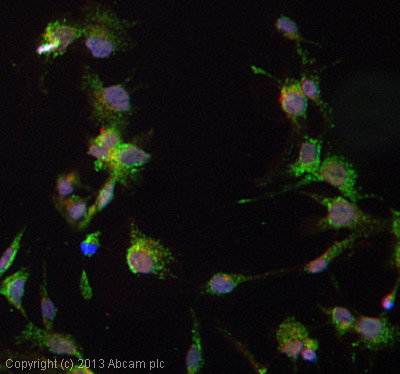
ICC/IF image of ab17438 stained HepG2 cells. The cells were 4% paraformaldehyde fixed (10 min) and then incubated in 1%BSA / 10% normal goat serum / 0.3M glycine in 0.1% PBS-Tween for 1h to permeabilise the cells and block non-specific protein-protein interactions. The cells were then incubated with the antibody (ab17438, 5µg/ml) overnight at +4°C. The secondary antibody (green) was ab96947, DyLight® 488 goat anti-chicken IgG (H+L) used at a 1/250 dilution for 1h. Alexa Fluor® 594 WGA was used to label plasma membranes (red) at a 1/200 dilution for 1h. DAPI was used to stain the cell nuclei (blue) at a concentration of 1.43µM.
