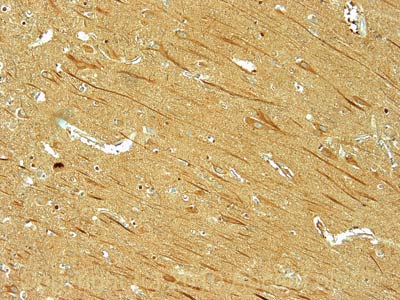
IHC image of 200kD Neurofilament Heavy staining in human hippocampus formalin fixed paraffin embedded tissue section, performed on a Leica BondTM system using the standard protocol F. The section was pre-treated using heat mediated antigen retrieval with sodium citrate buffer (pH6, epitope retrieval solution 1) for 20 mins. The section was then incubated with ab105453, 1µg/ml, for 15 mins at room temperature and detected using an HRP conjugated compact polymer system. DAB was used as the chromogen. The section was then counterstained with haematoxylin and mounted with DPX.
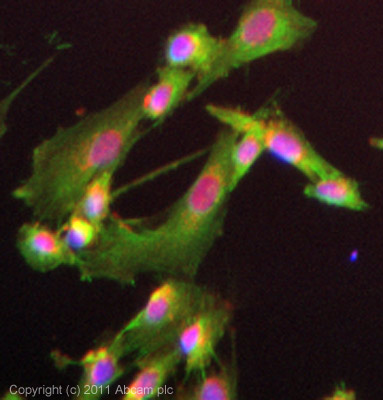
ICC/IF image of ab105453 stained SKNSH cells. The cells were 4% PFA fixed (10 min) and then incubated in 1%BSA / 10% normal goat serum / 0.3M glycine in 0.1% PBS-Tween for 1h to permeabilise the cells and block non-specific protein-protein interactions. The cells were then incubated with the antibody (ab105453, 5µg/ml) overnight at +4°C. The secondary antibody (green) was ab96899, DyLight® 488 goat anti-rabbit IgG (H+L) used at a 1/250 dilution for 1h. Alexa Fluor® 594 WGA was used to label plasma membranes (red) at a 1/200 dilution for 1h. DAPI was used to stain the cell nuclei (blue) at a concentration of 1.43µM. This antibody also gave a positive result in 100% methanol fixed (5 min) SKNSH cells at 5µg/ml.
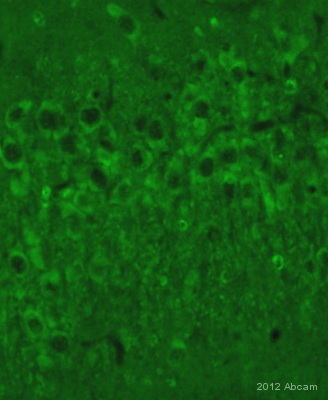
IHC-FoFr image of 200 kDa Neurofilament Heavy staining on rat hippocampus sections using ab105453 (/100).The sections used came from animals perfused fixed with Paraformaldehyde 4% with 15% of a solution of saturated picric acid, in phosphate buffer 0.1M. Following postfixation in the same fixative overnight, the spinal cord were cryoprotected in sucrose 30% overnight. Spinal cords were then cut using a cryostat and the immunostainings were performed using the ‘free floating’ technique.See Abreview


