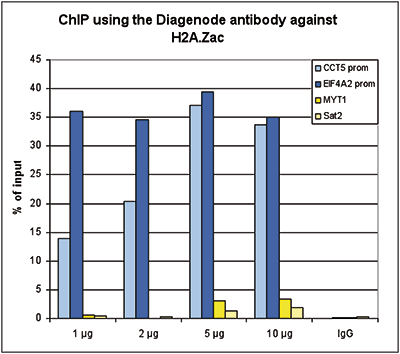
 Figure 1. ChIP results obtained with the Diagenode antibody directed against H2A.Zac
Figure 1. ChIP results obtained with the Diagenode antibody directed against H2A.Zac Figure 1A ChIP assays were performed using human HeLa cells, the Diagenode antibody against H2A.Zac (Cat. No. C15410202) and optimized PCR primer pairs for qPCR. ChIP was performed with the ““Auto Histone ChIP-seq” kit (Cat. No. AB-Auto02-A100) on the IP-Star automated system, using sheared chromatin from 1,000,000 cells. A titration consisting of 1, 2, 5 and 10 μg of antibody per ChIP experiment was analyzed. IgG (2 μg/IP) was used as a negative IP control. Figure 1B ChIP assays were performed using human K562 cells, the Diagenode antibody against H2A.Zac (Cat. No. C15410202) and optimized PCR primer sets for qPCR. ChIP was performed with the “iDeal ChIP-seq” kit (Cat. No. AB-001-0024) on sheared chromatin from 100,000 cells. A titration of the antibody consisting of 0.2, 0.5, 1 and 2 μg per ChIP experiment was analysed. IgG (1 μg/IP) was used as negative IP control. Quantitati
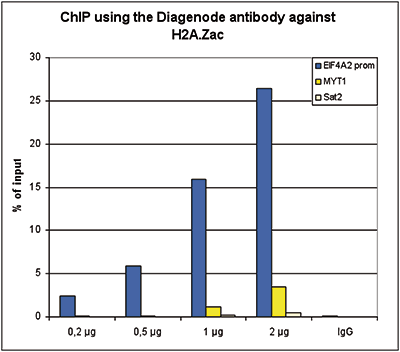 Figure 2. ChIP-seq results obtained with the Diagenode antibody directed against H2A.Zac
Figure 2. ChIP-seq results obtained with the Diagenode antibody directed against H2A.Zac ChIP was performed on sheared chromatin from 100,000 K562 cells using 1 μg the Diagenode antibody against H2A.Zac (Cat. No. C15410202) with the “iDeal ChIP-seq” kit (Cat. No. AB-001-0024) as described above. The IP’d DNA was subsequently analysed with an Illumina Genome Analyzer. Library preparation, cluster generation and sequencing were performed according to the manufacturer’s instructions. The 36 bp tags were aligned to the human genome using the ELAND algorithm. Figure 2 shows the peak distribution along the complete sequence and a 1.5 Mb region of the X-chromosome (figure 2A and B) and in two regions surrounding the EIF4A2 and CCT5 positive control genes, respectively (figure 2C and D).
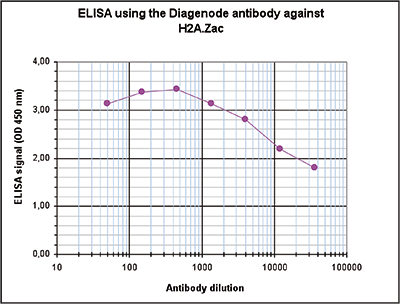
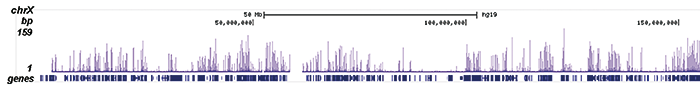 Figure 3. Determination of the antibody titer
Figure 3. Determination of the antibody titer To determine the titer of the antibody, an ELISA was performed using a serial dilution of the Diagenode antibody against H2A.Zac (Cat. No. C15410202). The antigen used was a peptide containing the histone modifications of interest. By plotting the absorbance against the antibody dilution (Figure 3), the titer of the antibody was estimated to be 1:56,600.
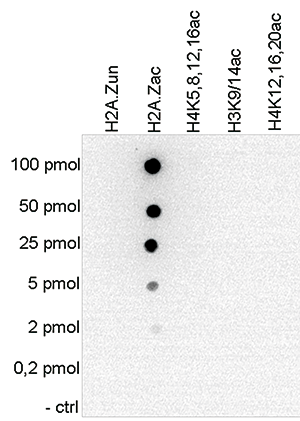
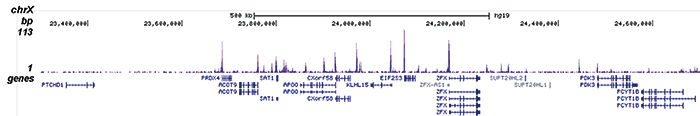 Figure 4. Cross reactivity tests using the Diagenode antibody directed against H2A.Zac
Figure 4. Cross reactivity tests using the Diagenode antibody directed against H2A.Zac To test the cross reactivity of the Diagenode antibody against H2A.Zac (Cat. No. C15410202), a Dot Blot analysis was performed with peptides containing different multiple acetylations and the unmodified H2A.Z. One hundred to 0.2 pmol of the respective peptides were spotted on a membrane. The antibody was used at a dilution of 1:20,000. Figure 4 shows a high specificity of the antibody for the modification of interest.
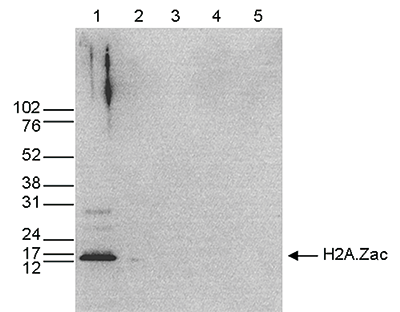
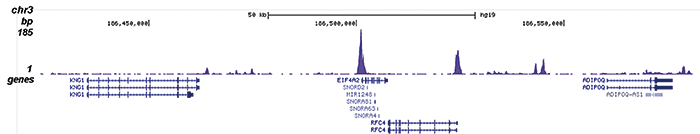 Figure 5. Western blot analysis using the Diagenode antibody directed against H2A.Zac
Figure 5. Western blot analysis using the Diagenode antibody directed against H2A.Zac Western blot was performed on whole cell extracts (25 μg, lane 1) from HeLa cells, and on 1 μg of recombinant histone H2A, H2B, H3 and H4 (lane 2, 3, 4 and 5, respectively) using the Diagenode antibody against H2A.Zac (Cat. No. C15410202). The antibody was diluted 1:1,000 in TBS-Tween containing 5% skimmed milk. The position of the protein of interest is indicated on the right, the marker (in kDa) is shown on the left.
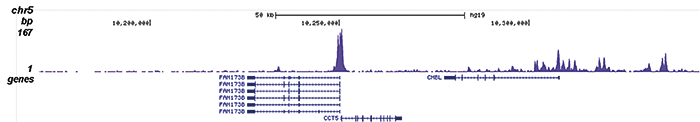 Figure 6. Immunofluorescence using the Diagenode antibody directed against H2A.Zac
Figure 6. Immunofluorescence using the Diagenode antibody directed against H2A.Zac HeLa cells were stained with the Diagenode antibody against H2A.Zac (Cat. No. C15410202) and with DAPI. Cells were fixed with 4% formaldehyde for 10’ and blocked with PBS/TX-100 containing 5% normal goat serum and 1% BSA. The cells were immunofluorescently labeled with the H2A.Zac antibody (left) diluted 1:500 in blocking solution followed by an anti-rabbit antibody conjugated to Alexa488. The middle panel shows staining of the nuclei with DAPI. A merge of the two stainings is shown on the right.