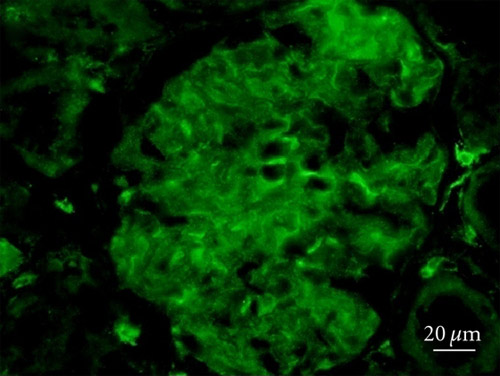
ab101883 staining Synaptopodin in diabetic rat kidney tissue by Immunohistochemistry (Frozen sections). Tissue was fixed with acetone, permeabilized with 1% Tween-20 in PBS and blocked with horse anti-mouse and goat anti-rabbit serum in PBS for at least 30 minutes. Samples were incubated with primary antibody (1/300 in diluent) for 60 minutes at room temperature. An AlexaFluor®488-conjugated goat anti-rabbit IgG was used as the secondary antibody.
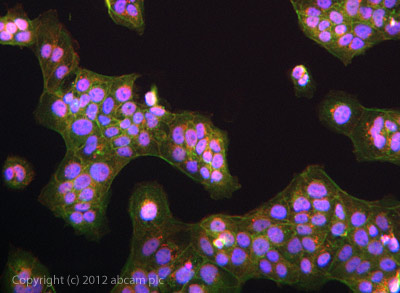
ab101883 stained JEG3 cells. The cells were 4% formaldehyde fixed (10 min) and then incubated in 1%BSA / 10% normal goat serum / 0.3M glycine in 0.1% PBS-Tween for 1h to permeabilise the cells and block non-specific protein-protein interactions. The cells were then incubated with the antibody ab101883 at 1 in 1000 dilution overnight at +4°C. The secondary antibody (green) was DyLight® 488 goat anti- rabbit (ab96899) IgG (H+L) used at a 1/1000 dilution for 1h. Alexa Fluor® 594 WGA was used to label plasma membranes (red) at a 1/200 dilution for 1h. DAPI was used to stain the cell nuclei (blue) at a concentration of 1.43µM.

