Anti-SCD1 antibody [CD.E10]
| Name | Anti-SCD1 antibody [CD.E10] |
|---|---|
| Supplier | Abcam |
| Catalog | ab19862 |
| Prices | $394.00 |
| Sizes | 100 µg |
| Host | Mouse |
| Clonality | Monoclonal |
| Isotype | IgG2b |
| Clone | CD.E10 |
| Applications | IHC-F IHC-P ICC/IF ICC/IF FC WB IP ELISA |
| Species Reactivities | Mouse, Rat, Human |
| Antigen | Recombinant full length protein (Human) |
| Description | Mouse Monoclonal |
| Gene | SCD |
| Conjugate | Unconjugated |
| Supplier Page | Shop |
Product images
Product References
Overexpression of stearoyl-CoA desaturase 1 in bone marrow mesenchymal stem cells - Overexpression of stearoyl-CoA desaturase 1 in bone marrow mesenchymal stem cells
Lu Y, Zhou Z, Tao J, Dou B, Gao M, Liu Y. Lipids Health Dis. 2014 Mar 20;13:53.
Long-term supranutritional supplementation with selenate decreases hyperglycemia - Long-term supranutritional supplementation with selenate decreases hyperglycemia
Wang C, Yang S, Zhang N, Mu Y, Ren H, Wang Y, Li K. PLoS One. 2014 Jul 1;9(7):e101315.
Hepatic and plasma sex differences in saturated and monounsaturated fatty acids - Hepatic and plasma sex differences in saturated and monounsaturated fatty acids
Marks KA, Kitson AP, Stark KD. Genes Nutr. 2013 May;8(3):317-27.
Proteomic and lipidomic signatures of lipid metabolism in NASH-associated - Proteomic and lipidomic signatures of lipid metabolism in NASH-associated
Muir K, Hazim A, He Y, Peyressatre M, Kim DY, Song X, Beretta L. Cancer Res. 2013 Aug 1;73(15):4722-31.
Fatty acid remodeling in cellular glycerophospholipids following the activation - Fatty acid remodeling in cellular glycerophospholipids following the activation
Robichaud PP, Boulay K, Munganyiki JE, Surette ME. J Lipid Res. 2013 Oct;54(10):2665-77.
Human cytomegalovirus infection induces adipocyte-like lipogenesis through - Human cytomegalovirus infection induces adipocyte-like lipogenesis through
Yu Y, Maguire TG, Alwine JC. J Virol. 2012 Mar;86(6):2942-9.
Induction of fatty acid synthesis is a key requirement for phagocytic - Induction of fatty acid synthesis is a key requirement for phagocytic
Ecker J, Liebisch G, Englmaier M, Grandl M, Robenek H, Schmitz G. Proc Natl Acad Sci U S A. 2010 Apr 27;107(17):7817-22. doi:
Abrogation of de novo lipogenesis by stearoyl-CoA desaturase 1 inhibition - Abrogation of de novo lipogenesis by stearoyl-CoA desaturase 1 inhibition
Fritz V, Benfodda Z, Rodier G, Henriquet C, Iborra F, Avances C, Allory Y, de la Taille A, Culine S, Blancou H, Cristol JP, Michel F, Sardet C, Fajas L. Mol Cancer Ther. 2010 Jun;9(6):1740-54.
Lower SCD expression in dendritic cells compared to macrophages leads to membrane - Lower SCD expression in dendritic cells compared to macrophages leads to membrane
Ecker J, Liebisch G, Grandl M, Schmitz G. Immunobiology. 2010 Sep-Oct;215(9-10):748-55.
Susceptibility of pancreatic beta cells to fatty acids is regulated by - Susceptibility of pancreatic beta cells to fatty acids is regulated by
Hellemans KH, Hannaert JC, Denys B, Steffensen KR, Raemdonck C, Martens GA, Van Veldhoven PP, Gustafsson JA, Pipeleers D. PLoS One. 2009 Sep 29;4(9):e7266.

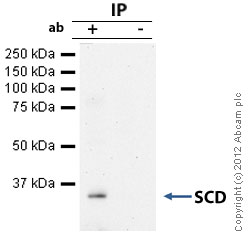
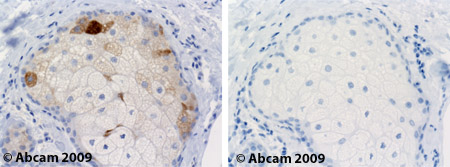
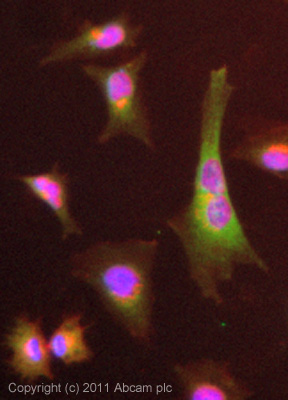
![Overlay histogram showing HepG2 cells stained with ab19862 (red line). The cells were fixed with 4% paraformaldehyde (10 min) and then permeabilized with 0.1% PBS-Tween for 20 min. The cells were then incubated in 1x PBS / 10% normal goat serum / 0.3M glycine to block non-specific protein-protein interactions followed by the antibody (ab19862, 1µg/1x106 cells) for 30 min at 22ºC. The secondary antibody used was DyLight® 488 goat anti-mouse IgG (H+L) (ab96879) at 1/500 dilution for 30 min at 22ºC. Isotype control antibody (black line) was mouse IgG2b [PLPV219] (ab91366, 2µg/1x106 cells) used under the same conditions. Acquisition of >5,000 events was performed. This antibody gave a positive signal in HepG2 cells fixed with 80% methanol (5 min)/permeabilized in 0.1% PBS-Tween used under the same conditions.](http://www.bioprodhub.com/system/product_images/ab_products/2/sub_4/27280_SCD-Primary-antibodies-ab19862-4.jpg)