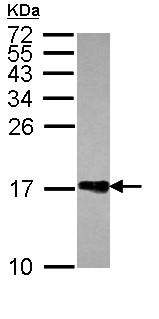
Anti-Histone H2A.Z antibody - ChIP Grade (ab97941) at 1/1000 dilution + Jurkat whole cell lysate at 30 µg
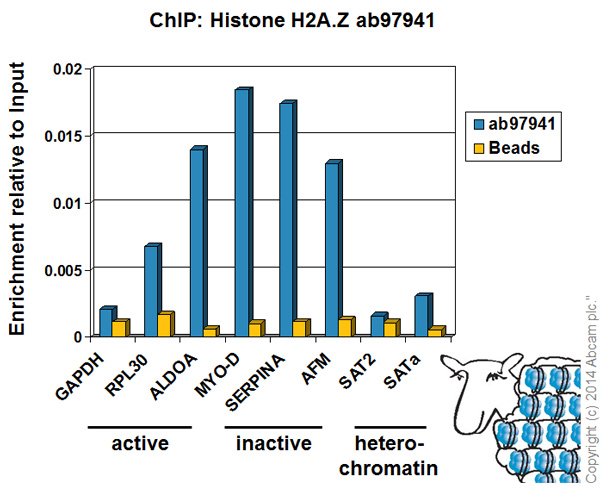
Chromatin was prepared from HeLa cells according to the Abcam X-ChIP protocol. Cells were fixed with formaldehyde for 10 minutes. The ChIP was performed with 25µg of chromatin, 2µg of ab97941 (blue), and 20µl of Protein A/G sepharose beads. No antibody was added to the beads control (yellow). The immunoprecipitated DNA was quantified by real time PCR (Taqman approach for active and inactive loci, Sybr green approach for heterochromatic loci). Primers and probes are located in the first kb of the transcribed region.
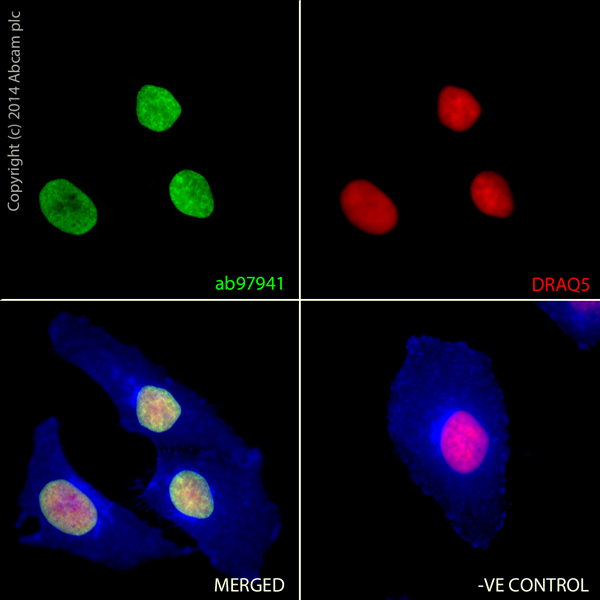
ab97941 staining Histone H2A.Z in HeLa cells. The cells were fixed with 100% methanol (5min) and then blocked in 1% BSA/10% normal goat serum/0.3M glycine in 0.1%PBS-Tween for 1h. The cells were then incubated with ab97941 at 1μg/ml overnight at +4°C, followed by a further incubation at room temperature for 1h with an AlexaFluor®488 Goat anti-Rabbit secondary (ab150077) at 2 μg/ml (shown in green). AlexaFluor®350 WGA was used at a 1/200 dilution and incubated for 1h with the cells, to label plasma membranes (shown in blue). Nuclear DNA was labelled in red with 1.25 μM DRAQ5™ (ab108410), which was added to the secondary antibody mixture. A secondary only negative control is displayed, which indicates that the Histone H2A.Z staining observed is due to primary antibody specificity and not to unspecific binding of the secondary antibody to the cells.


