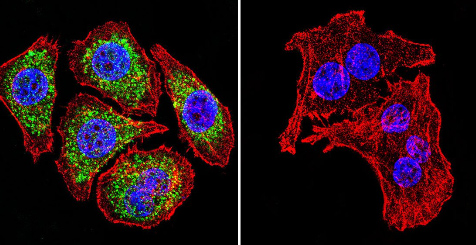
Immunocytochemistry/Immunofluorescence analysis of U251 cells labeling Glucocorticoid Receptor alpha (green) with ab3580 at 1/100. F-Actin staining with Phalloidin (red) and nuclei with DAPI (blue). Cells were fixed with formaldehyde and incubated with the primary antibody overnight at 4°C. A DyLight 488-conjugated secondary antibody was used. 60X magnification. Right - negative control.
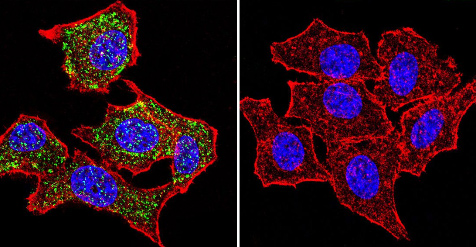
Immunocytochemistry/Immunofluorescence analysis of HeLa cells labeling Glucocorticoid Receptor alpha (green) with ab3580 at 1/100. F-Actin staining with Phalloidin (red) and nuclei with DAPI (blue). Cells were fixed with formaldehyde and incubated with the primary antibody overnight at 4°C. A DyLight 488-conjugated secondary antibody was used. 60X magnification. Right - negative control.
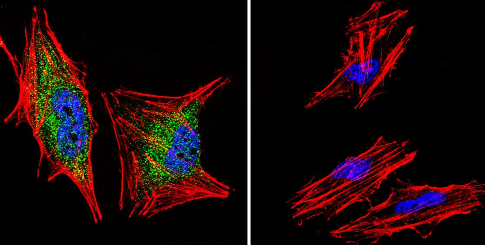
Immunocytochemistry/Immunofluorescence analysis of A2058 cells labeling Glucocorticoid Receptor alpha (green) with ab3580 at 1/200. F-Actin staining with Phalloidin (red) and nuclei with DAPI (blue). Cells were fixed with formaldehyde and incubated with the primary antibody overnight at 4°C. A DyLight 488-conjugated secondary antibody was used. 60X magnification. Right - negative control.
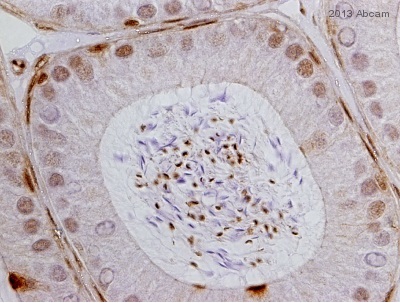
ab3580 staining Glucocorticoid Receptor alpha in epididymis tissue sections by Immunohistochemistry (IHC-P - paraformaldehyde-fixed, paraffin-embedded sections). Tissue was fixed with Bouin's solution and blocked with 1.5% serum for 30 minutes at 25°C; antigen retrieval was by heat mediation in a citrate buffer. Samples were incubated with primary antibody (1/1000 in blocking buffer) for 14 hours at 4°C. ab6721 Goat anti-rabbit HRP (1/200) was used as the secondary antibody.See Abreview

ICC/IF image of ab3580 stained Hek293 cells. The cells were 4% PFA fixed (10 min) and then incubated in 1%BSA / 10% normal Goat serum / 0.3M glycine in 0.1% PBS-Tween for 1h to permeabilise the cells and block non-specific protein-protein interactions. The cells were then incubated with the antibody (ab3580, 1µg/ml) overnight at +4°C. The secondary antibody (green) was Alexa Fluor® 488 Goat anti-Rabbit IgG (H+L) used at a 1/1000 dilution for 1h. Alexa Fluor® 594 WGA was used to label plasma membranes (red) at a 1/200 dilution for 1h. DAPI was used to stain the cell nuclei (blue) at a concentration of 1.43µM.

ab3580 staining glucocorticoid receptor in serum starved HeLa cells treated with rosiglitazone (ab120762), by ICC/IF. Changes in localization of glucocorticoid receptor (translocation from cytoplasm to nucleous) correlates with increased concentration of rosiglitazone, as described in literature.The cells were incubated at 37°C for 1h in media containing different concentrations of ab120762 (rosiglitazone) in DMSO, fixed with 4% formaldehyde for 10 minutes at room temperature and blocked with PBS containing 10% goat serum, 0.3 M glycine, 1% BSA and 0.1% tween for 2h at room temperature. Staining of the treated cells with ab3580 (5 µg/ml) was performed overnight at 4°C in PBS containing 1% BSA and 0.1% tween. A DyLight 488 goat anti-rabbit polyclonal antibody (ab96899) at 1/250 dilution was used as the secondary antibody. Nuclei were counterstained with DAPI and are shown in blue.

ab3580 staining glucocorticoid receptor in serum starved HeLa cells treated with rosiglitazone (ab120762), by ICC/IF. Changes in localization of glucocorticoid receptor (translocation from cytoplasm to nucleous) correlates with increased concentration of rosiglitazone, as described in literature.The cells were incubated at 37°C for 1h in media containing different concentrations of ab120762 (rosiglitazone) in DMSO, fixed with 4% formaldehyde for 10 minutes at room temperature and blocked with PBS containing 10% goat serum, 0.3 M glycine, 1% BSA and 0.1% tween for 2h at room temperature. Staining of the treated cells with ab3580 (5 µg/ml) was performed overnight at 4°C in PBS containing 1% BSA and 0.1% tween. A DyLight 488 goat anti-rabbit polyclonal antibody (ab96899) at 1/250 dilution was used as the secondary antibody. Nuclei were counterstained with DAPI and are shown in blue.

ab3580 staining glucocorticoid receptor in serum starved HeLa cells treated with rosiglitazone (ab120762), by ICC/IF. Changes in localization of glucocorticoid receptor (translocation from cytoplasm to nucleous) correlates with increased concentration of rosiglitazone, as described in literature.The cells were incubated at 37°C for 1h in media containing different concentrations of ab120762 (rosiglitazone) in DMSO, fixed with 4% formaldehyde for 10 minutes at room temperature and blocked with PBS containing 10% goat serum, 0.3 M glycine, 1% BSA and 0.1% tween for 2h at room temperature. Staining of the treated cells with ab3580 (5 µg/ml) was performed overnight at 4°C in PBS containing 1% BSA and 0.1% tween. A DyLight 488 goat anti-rabbit polyclonal antibody (ab96899) at 1/250 dilution was used as the secondary antibody. Nuclei were counterstained with DAPI and are shown in blue.

ab3580 staining glucocorticoid receptor in serum starved HeLa cells treated with rosiglitazone (ab120762), by ICC/IF. Changes in localization of glucocorticoid receptor (translocation from cytoplasm to nucleous) correlates with increased concentration of rosiglitazone, as described in literature.The cells were incubated at 37°C for 1h in media containing different concentrations of ab120762 (rosiglitazone) in DMSO, fixed with 4% formaldehyde for 10 minutes at room temperature and blocked with PBS containing 10% goat serum, 0.3 M glycine, 1% BSA and 0.1% tween for 2h at room temperature. Staining of the treated cells with ab3580 (5 µg/ml) was performed overnight at 4°C in PBS containing 1% BSA and 0.1% tween. A DyLight 488 goat anti-rabbit polyclonal antibody (ab96899) at 1/250 dilution was used as the secondary antibody. Nuclei were counterstained with DAPI and are shown in blue.

ab3580 (1µg/ml) staining glucocorticoid receptor alpha in human hippocampus using an automated system (DAKO Autostainer Plus). Using this protocol there is cytoplasmic staining in the neuropil and blood vessel smooth muscle.Sections were rehydrated and antigen retrieved with the Dako 3 in 1 AR buffer EDTA pH 9.0 in a DAKO PT Link. Slides were peroxidase blocked in 3% H2O2 in methanol for 10 mins. They were then blocked with Dako Protein block for 10 minutes (containing casein 0.25% in PBS) then incubated with primary antibody for 20 min and detected with Dako Envision Flex amplification kit for 30 minutes. Colorimetric detection was completed with Diaminobenzidine for 5 minutes. Slides were counterstained with Haematoxylin and coverslipped under DePeX. Please note that, for manual staining, optimization of primary antibody concentration and incubation time is recommended. Signal amplification may be required.

ab3580 (1µg/ml) staining glucocorticoid receptor alpha in human hippocampus using an automated system (DAKO Autostainer Plus). Using this protocol there is cytoplasmic staining in the neuropil and blood vessel smooth muscle.Sections were rehydrated and antigen retrieved with the Dako 3 in 1 AR buffer EDTA pH 9.0 in a DAKO PT Link. Slides were peroxidase blocked in 3% H2O2 in methanol for 10 mins. They were then blocked with Dako Protein block for 10 minutes (containing casein 0.25% in PBS) then incubated with primary antibody for 20 min and detected with Dako Envision Flex amplification kit for 30 minutes. Colorimetric detection was completed with Diaminobenzidine for 5 minutes. Slides were counterstained with Haematoxylin and coverslipped under DePeX. Please note that, for manual staining, optimization of primary antibody concentration and incubation time is recommended. Signal amplification may be required.

ab3580 (1µg/ml) staining glucocorticoid receptor alpha in human hippocampus using an automated system (DAKO Autostainer Plus). Using this protocol there is cytoplasmic staining in the neuropil and blood vessel smooth muscle.Sections were rehydrated and antigen retrieved with the Dako 3 in 1 AR buffer EDTA pH 9.0 in a DAKO PT Link. Slides were peroxidase blocked in 3% H2O2 in methanol for 10 mins. They were then blocked with Dako Protein block for 10 minutes (containing casein 0.25% in PBS) then incubated with primary antibody for 20 min and detected with Dako Envision Flex amplification kit for 30 minutes. Colorimetric detection was completed with Diaminobenzidine for 5 minutes. Slides were counterstained with Haematoxylin and coverslipped under DePeX. Please note that, for manual staining, optimization of primary antibody concentration and incubation time is recommended. Signal amplification may be required.

ab3580 (1µg/ml) staining glucocorticoid receptor alpha in human hippocampus using an automated system (DAKO Autostainer Plus). Using this protocol there is cytoplasmic staining in the neuropil and blood vessel smooth muscle.Sections were rehydrated and antigen retrieved with the Dako 3 in 1 AR buffer EDTA pH 9.0 in a DAKO PT Link. Slides were peroxidase blocked in 3% H2O2 in methanol for 10 mins. They were then blocked with Dako Protein block for 10 minutes (containing casein 0.25% in PBS) then incubated with primary antibody for 20 min and detected with Dako Envision Flex amplification kit for 30 minutes. Colorimetric detection was completed with Diaminobenzidine for 5 minutes. Slides were counterstained with Haematoxylin and coverslipped under DePeX. Please note that, for manual staining, optimization of primary antibody concentration and incubation time is recommended. Signal amplification may be required.

Immunohistochemistry was performed on both normal and cancer biopsies of deparaffinized Human cervical carcinoma tissues. To expose target proteins heat induced antigen retrieval was performed using 10mM sodium citrate (pH6.0) buffer microwaved for 8-15 minutes. Following antigen retrieval tissues were blocked in 3% BSA-PBS for 30 minutes at room temperature. Tissues were then probed at a dilution of 1:20 with a rabbit polyclonal antibody recognizing Glucocorticoid Receptor alpha ab3580 or without primary antibody (negative control) overnight at 4°C in a humidified chamber. Tissues were washed extensively with PBST and endogenous peroxidase activity was quenched with a peroxidase suppressor. Detection was performed using a biotin-conjugated secondary antibody and SA-HRP followed by colorimetric detection using DAB. Tissues were counterstained with hematoxylin and prepped for mounting.

Immunohistochemistry was performed on both normal and cancer biopsies of deparaffinized Human cervical carcinoma tissues. To expose target proteins heat induced antigen retrieval was performed using 10mM sodium citrate (pH6.0) buffer microwaved for 8-15 minutes. Following antigen retrieval tissues were blocked in 3% BSA-PBS for 30 minutes at room temperature. Tissues were then probed at a dilution of 1:20 with a rabbit polyclonal antibody recognizing Glucocorticoid Receptor alpha ab3580 or without primary antibody (negative control) overnight at 4°C in a humidified chamber. Tissues were washed extensively with PBST and endogenous peroxidase activity was quenched with a peroxidase suppressor. Detection was performed using a biotin-conjugated secondary antibody and SA-HRP followed by colorimetric detection using DAB. Tissues were counterstained with hematoxylin and prepped for mounting.

Immunohistochemistry was performed on both normal and cancer biopsies of deparaffinized Human cervical carcinoma tissues. To expose target proteins heat induced antigen retrieval was performed using 10mM sodium citrate (pH6.0) buffer microwaved for 8-15 minutes. Following antigen retrieval tissues were blocked in 3% BSA-PBS for 30 minutes at room temperature. Tissues were then probed at a dilution of 1:20 with a rabbit polyclonal antibody recognizing Glucocorticoid Receptor alpha ab3580 or without primary antibody (negative control) overnight at 4°C in a humidified chamber. Tissues were washed extensively with PBST and endogenous peroxidase activity was quenched with a peroxidase suppressor. Detection was performed using a biotin-conjugated secondary antibody and SA-HRP followed by colorimetric detection using DAB. Tissues were counterstained with hematoxylin and prepped for mounting.

Immunohistochemistry was performed on both normal and cancer biopsies of deparaffinized Human cervical carcinoma tissues. To expose target proteins heat induced antigen retrieval was performed using 10mM sodium citrate (pH6.0) buffer microwaved for 8-15 minutes. Following antigen retrieval tissues were blocked in 3% BSA-PBS for 30 minutes at room temperature. Tissues were then probed at a dilution of 1:20 with a rabbit polyclonal antibody recognizing Glucocorticoid Receptor alpha ab3580 or without primary antibody (negative control) overnight at 4°C in a humidified chamber. Tissues were washed extensively with PBST and endogenous peroxidase activity was quenched with a peroxidase suppressor. Detection was performed using a biotin-conjugated secondary antibody and SA-HRP followed by colorimetric detection using DAB. Tissues were counterstained with hematoxylin and prepped for mounting.

Immunohistochemistry was performed on both normal and cancer biopsies of deparaffinized Human heart tissue tissues. To expose target proteins heat induced antigen retrieval was performed using 10mM sodium citrate (pH6.0) buffer microwaved for 8-15 minutes. Following antigen retrieval tissues were blocked in 3% BSA-PBS for 30 minutes at room temperature. Tissues were then probed at a dilution of 1:100 with a rabbit polyclonal antibody recognizing Glucocorticoid Receptor alpha ab3580 or without primary antibody (negative control) overnight at 4°C in a humidified chamber. Tissues were washed extensively with PBST and endogenous peroxidase activity was quenched with a peroxidase suppressor. Detection was performed using a biotin-conjugated secondary antibody and SA-HRP followed by colorimetric detection using DAB. Tissues were counterstained with hematoxylin and prepped for mounting.

Immunohistochemistry was performed on both normal and cancer biopsies of deparaffinized Human heart tissue tissues. To expose target proteins heat induced antigen retrieval was performed using 10mM sodium citrate (pH6.0) buffer microwaved for 8-15 minutes. Following antigen retrieval tissues were blocked in 3% BSA-PBS for 30 minutes at room temperature. Tissues were then probed at a dilution of 1:100 with a rabbit polyclonal antibody recognizing Glucocorticoid Receptor alpha ab3580 or without primary antibody (negative control) overnight at 4°C in a humidified chamber. Tissues were washed extensively with PBST and endogenous peroxidase activity was quenched with a peroxidase suppressor. Detection was performed using a biotin-conjugated secondary antibody and SA-HRP followed by colorimetric detection using DAB. Tissues were counterstained with hematoxylin and prepped for mounting.

Immunohistochemistry was performed on both normal and cancer biopsies of deparaffinized Human heart tissue tissues. To expose target proteins heat induced antigen retrieval was performed using 10mM sodium citrate (pH6.0) buffer microwaved for 8-15 minutes. Following antigen retrieval tissues were blocked in 3% BSA-PBS for 30 minutes at room temperature. Tissues were then probed at a dilution of 1:100 with a rabbit polyclonal antibody recognizing Glucocorticoid Receptor alpha ab3580 or without primary antibody (negative control) overnight at 4°C in a humidified chamber. Tissues were washed extensively with PBST and endogenous peroxidase activity was quenched with a peroxidase suppressor. Detection was performed using a biotin-conjugated secondary antibody and SA-HRP followed by colorimetric detection using DAB. Tissues were counterstained with hematoxylin and prepped for mounting.

Immunohistochemistry was performed on both normal and cancer biopsies of deparaffinized Human heart tissue tissues. To expose target proteins heat induced antigen retrieval was performed using 10mM sodium citrate (pH6.0) buffer microwaved for 8-15 minutes. Following antigen retrieval tissues were blocked in 3% BSA-PBS for 30 minutes at room temperature. Tissues were then probed at a dilution of 1:100 with a rabbit polyclonal antibody recognizing Glucocorticoid Receptor alpha ab3580 or without primary antibody (negative control) overnight at 4°C in a humidified chamber. Tissues were washed extensively with PBST and endogenous peroxidase activity was quenched with a peroxidase suppressor. Detection was performed using a biotin-conjugated secondary antibody and SA-HRP followed by colorimetric detection using DAB. Tissues were counterstained with hematoxylin and prepped for mounting.

Immunohistochemistry was performed on both normal and cancer biopsies of deparaffinized Human tonsil tissue tissues. To expose target proteins heat induced antigen retrieval was performed using 10mM sodium citrate (pH6.0) buffer microwaved for 8-15 minutes. Following antigen retrieval tissues were blocked in 3% BSA-PBS for 30 minutes at room temperature. Tissues were then probed at a dilution of 1:50 with a rabbit polyclonal antibody recognizing Glucocorticoid Receptor alpha ab3580 or without primary antibody (negative control) overnight at 4°C in a humidified chamber. Tissues were washed extensively with PBST and endogenous peroxidase activity was quenched with a peroxidase suppressor. Detection was performed using a biotin-conjugated secondary antibody and SA-HRP followed by colorimetric detection using DAB. Tissues were counterstained with hematoxylin and prepped for mounting.

Immunohistochemistry was performed on both normal and cancer biopsies of deparaffinized Human tonsil tissue tissues. To expose target proteins heat induced antigen retrieval was performed using 10mM sodium citrate (pH6.0) buffer microwaved for 8-15 minutes. Following antigen retrieval tissues were blocked in 3% BSA-PBS for 30 minutes at room temperature. Tissues were then probed at a dilution of 1:50 with a rabbit polyclonal antibody recognizing Glucocorticoid Receptor alpha ab3580 or without primary antibody (negative control) overnight at 4°C in a humidified chamber. Tissues were washed extensively with PBST and endogenous peroxidase activity was quenched with a peroxidase suppressor. Detection was performed using a biotin-conjugated secondary antibody and SA-HRP followed by colorimetric detection using DAB. Tissues were counterstained with hematoxylin and prepped for mounting.

Immunohistochemistry was performed on both normal and cancer biopsies of deparaffinized Human tonsil tissue tissues. To expose target proteins heat induced antigen retrieval was performed using 10mM sodium citrate (pH6.0) buffer microwaved for 8-15 minutes. Following antigen retrieval tissues were blocked in 3% BSA-PBS for 30 minutes at room temperature. Tissues were then probed at a dilution of 1:50 with a rabbit polyclonal antibody recognizing Glucocorticoid Receptor alpha ab3580 or without primary antibody (negative control) overnight at 4°C in a humidified chamber. Tissues were washed extensively with PBST and endogenous peroxidase activity was quenched with a peroxidase suppressor. Detection was performed using a biotin-conjugated secondary antibody and SA-HRP followed by colorimetric detection using DAB. Tissues were counterstained with hematoxylin and prepped for mounting.

Immunohistochemistry was performed on both normal and cancer biopsies of deparaffinized Human tonsil tissue tissues. To expose target proteins heat induced antigen retrieval was performed using 10mM sodium citrate (pH6.0) buffer microwaved for 8-15 minutes. Following antigen retrieval tissues were blocked in 3% BSA-PBS for 30 minutes at room temperature. Tissues were then probed at a dilution of 1:50 with a rabbit polyclonal antibody recognizing Glucocorticoid Receptor alpha ab3580 or without primary antibody (negative control) overnight at 4°C in a humidified chamber. Tissues were washed extensively with PBST and endogenous peroxidase activity was quenched with a peroxidase suppressor. Detection was performed using a biotin-conjugated secondary antibody and SA-HRP followed by colorimetric detection using DAB. Tissues were counterstained with hematoxylin and prepped for mounting.
























